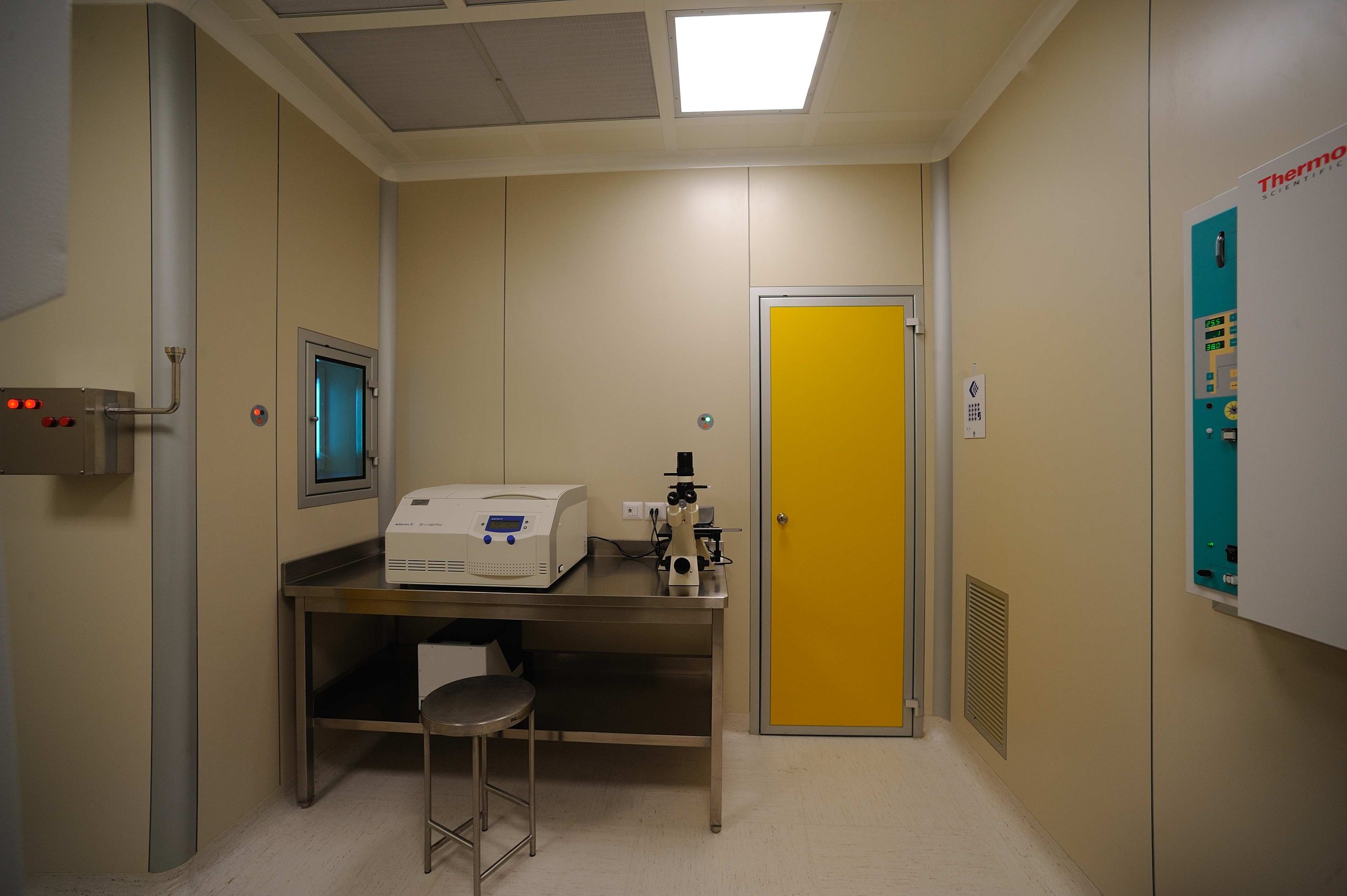
Today scientific research makes it possible to prevent or correct various pathologies through cell, gene therapy, and tissue engineering. Processes and environments must comply with GMP - Good Manufacturing Practices standards. Acotec Turnkey Division, in Italy, was a pioneer in design and construction of AIFA validated environments for advanced therapies and authorized to operate as pharmaceutical laboratories. GMP laboratories design and creation of biological containment laboratories are consolidated skills for Acotec with many references in cell-factories construction.